Imidazole ring formation and tertiary amine cleavage upon base-mediated nucleophilic substitution in 1,1,3-trichloro-1H-isoindole with α-(N-alkylamino) ketones | SpringerLink
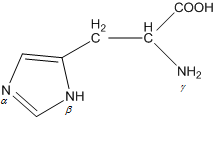
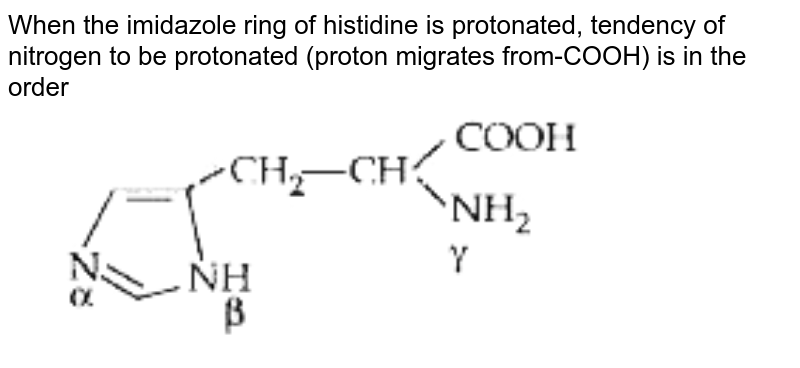
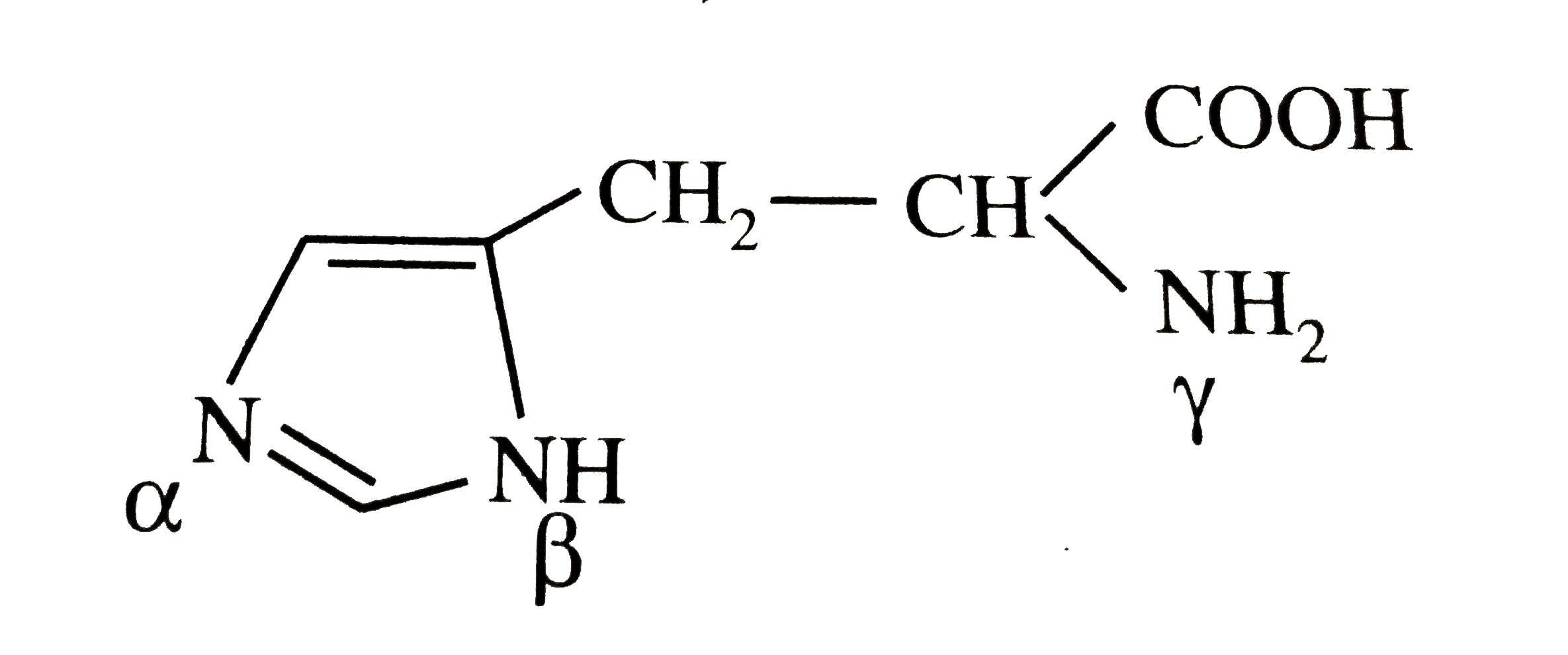
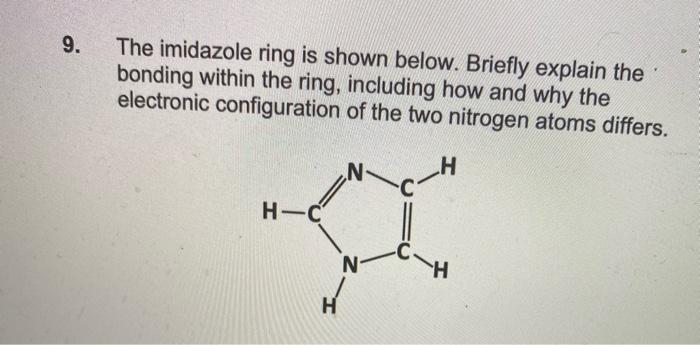
When the imidazole ring of Histidine is protonated, the tendency of nitrogen to be protonated (proton migrates from-COOH) is in the order?\n \n \n \n \n A. $\\beta \\gamma \\alpha $B. $\\gamma \\

SCHEME 5 Imidazole ring-opening of guaninium salt 4d in alkaline solution | Download Scientific Diagram

Applications of Purine Ring Opening in the Synthesis of Imidazole, Pyrimidine, and New Purine Derivatives - Leškovskis - 2021 - European Journal of Organic Chemistry - Wiley Online Library

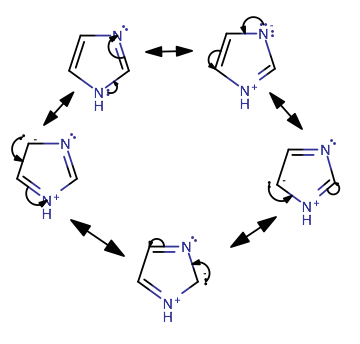
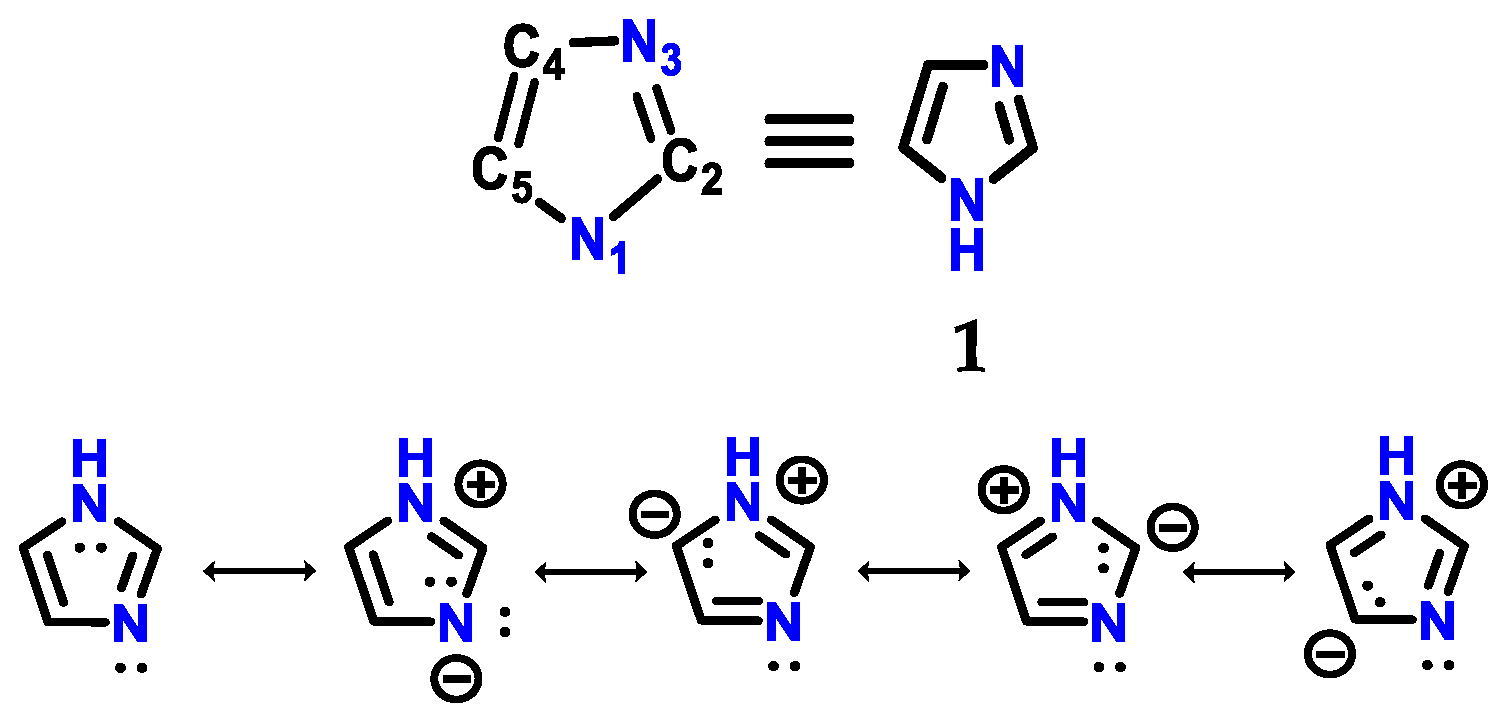
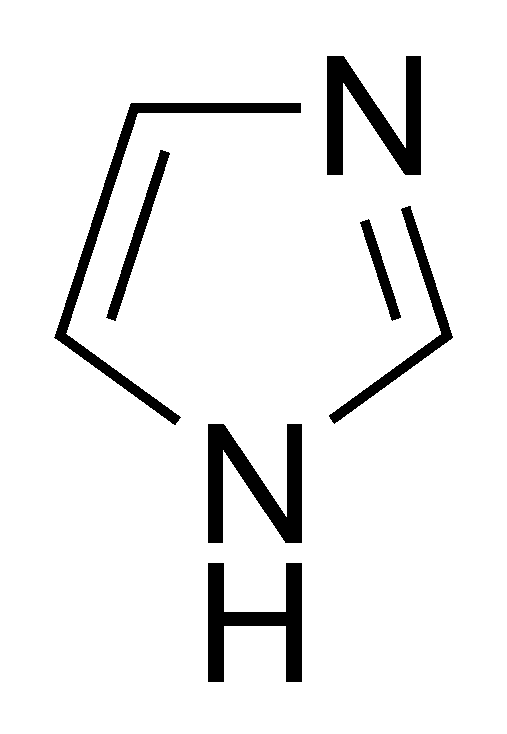
organic chemistry - Why isn't this resonance possible in an imidazole ring? - Chemistry Stack Exchange

Figure 2 from Imidazole ring-opened DNA purines and their biological significance. | Semantic Scholar
Molecules | Free Full-Text | Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry


![PDF] Imidazole ring-opened DNA purines and their biological significance. | Semantic Scholar PDF] Imidazole ring-opened DNA purines and their biological significance. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/693b6322da2d6bd2b6277f6bac31b731894d18bf/1-Figure1-1.png)